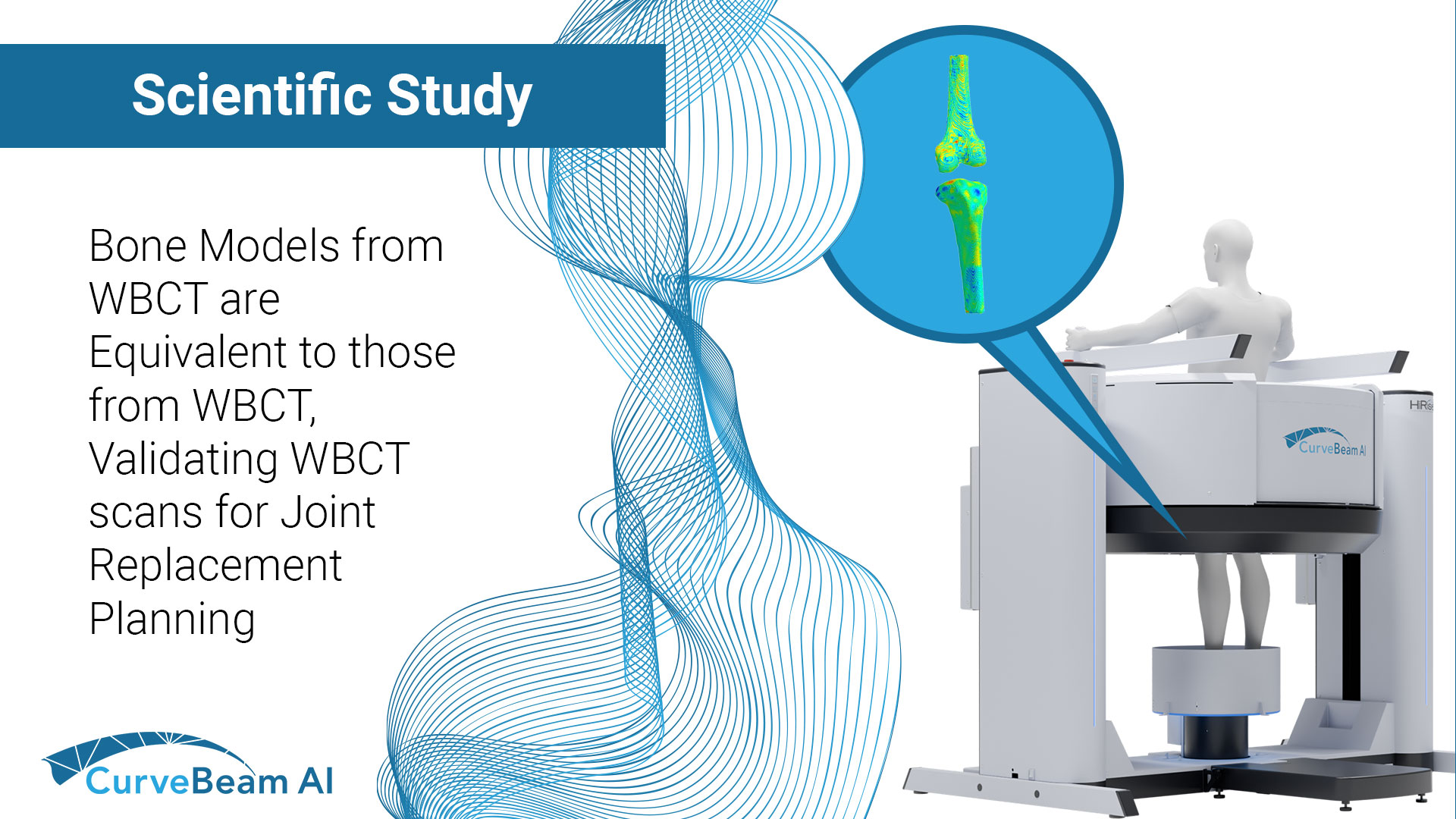
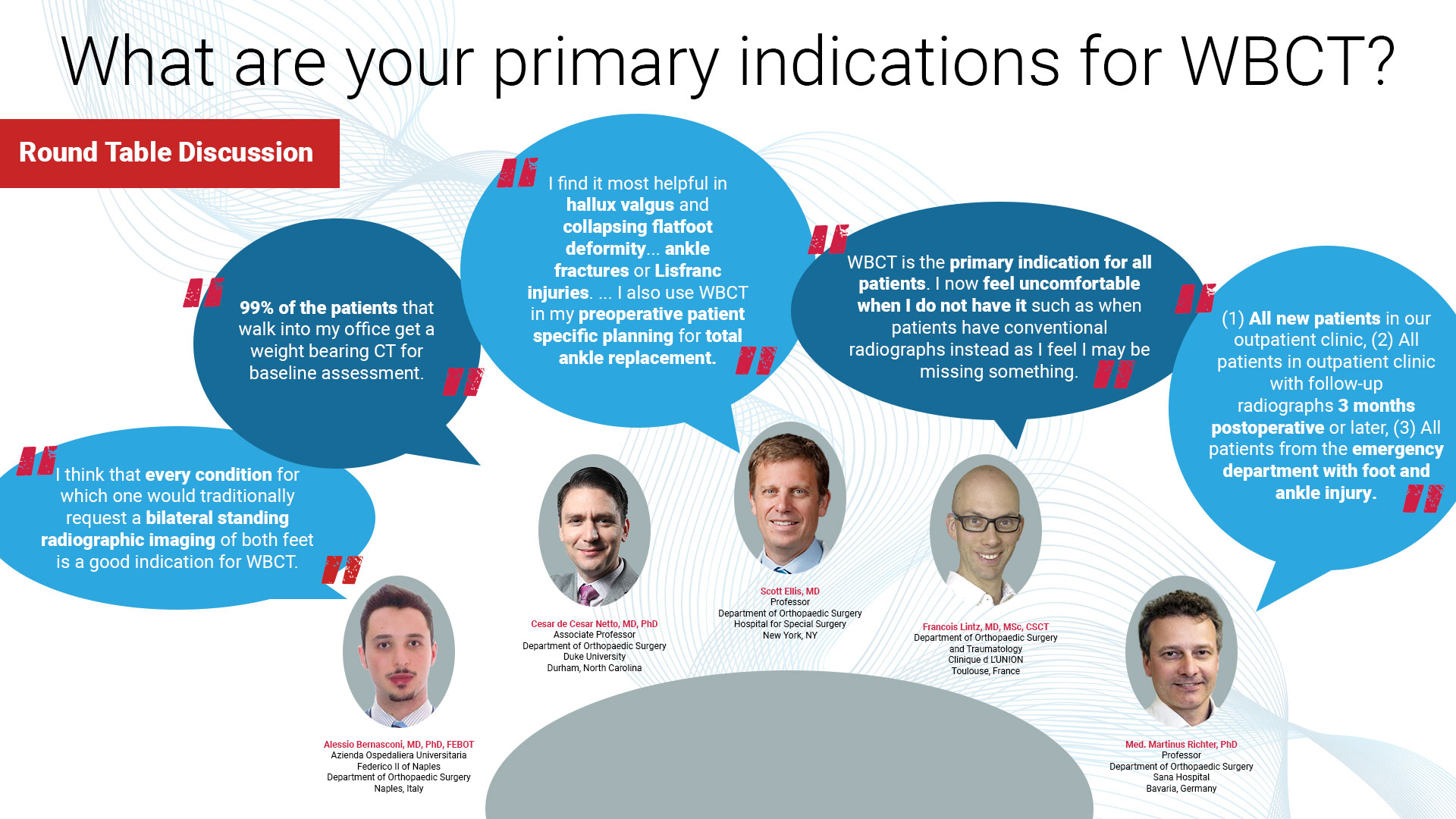
AOFAS Winter Meeting 2026
WBCT on the Program Thursday, January 22 11:10 am – 12:30 pm Symposium 2: Diagnostic Challenges Weightbearing CT and Treatment of Ankle Arthritis Amy L. Lenz, PhD Saturday, January 24 9:15 – 10:15 am Paper Session 2 Moderators: James…