Half of women over age 50 in the U.S. will experience a fragility fracture in their lifetime1.
Hip fractures in older women have higher one-year mortality rates than breast cancer2.
Up to half of hip fractures and most wrist fractures occur in women with osteopenia3,4. Osteopenia is much more common than osteoporosis5.
More than 10% of “osteoporosis-related” fractures occur in those with normal or “only” osteopenic BMD6.
What if there was potentially a better way to monitor for the progression of osteopenia and osteoporosis over time to help prevent fragility fractures?
“[Orthopedic] surgeons are uniquely positioned to identify patients at risk of underlying bone health problems during routine practice. …Opportunistic CT [are expected to] lessen the cost of screening, fracture treatment and prevention.” 7
In Development: Convenient, Automated Bone Microstructure Evaluation

InReach fits seamlessly into imaging centers, hospitals, and even the point-of-care setting, improving patient access for screening and monitoring therapy.

OssView is in development to work seamlessly with InReach to provide consistent images, ensuring reliable AI-aided evaluation.
Bone Fragility Assessment
CurveBeam AI is committed to advancing automated imaging tools to evaluate bone microstructure.
In OssView, Region of Interest selection will be automated to ensure consistency.

InReach* scans the wrist.
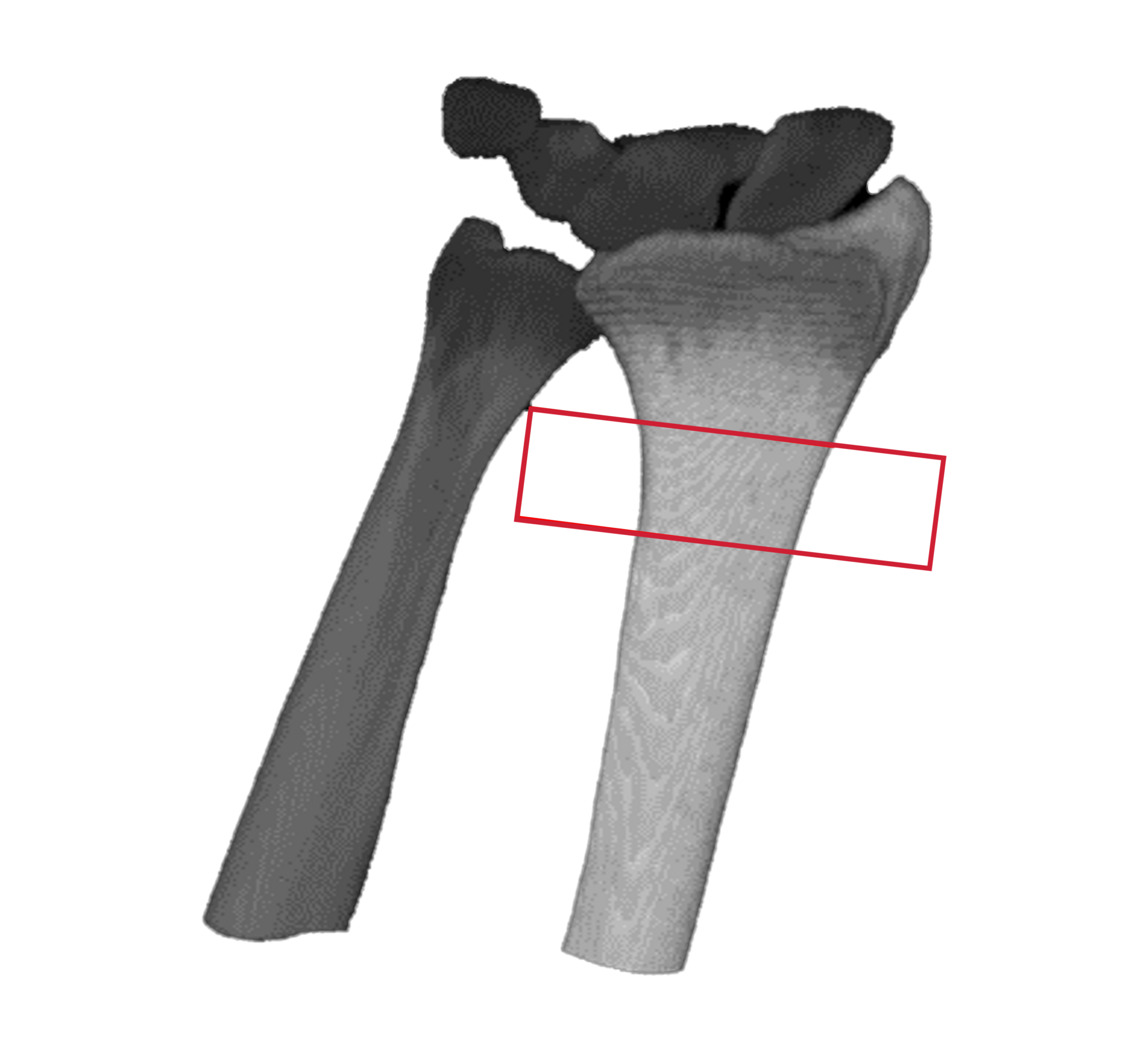
Deep Learning AI will help extract & identify bones.
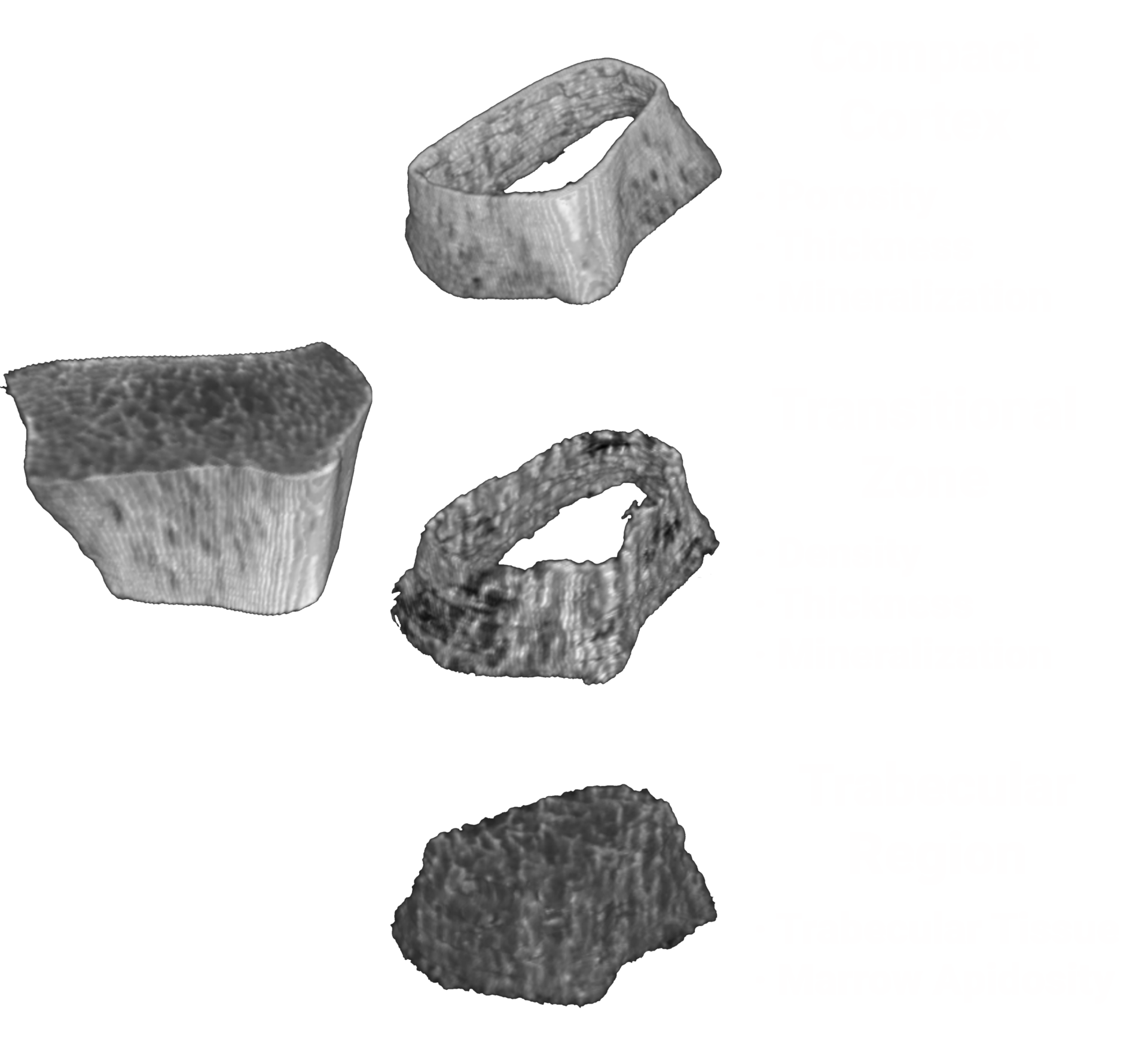
Bone microstructure will be segmented.
This technology is currently under development and is not yet cleared or approved by the U.S. FDA. Availability is subject to completion of development and applicable regulatory approvals. Regulatory approval and availability may vary by country.
RECEIVE MORE INFORMATION ABOUT THE HR-pQCT

Automated Bone Fragility Analysis with OssView®
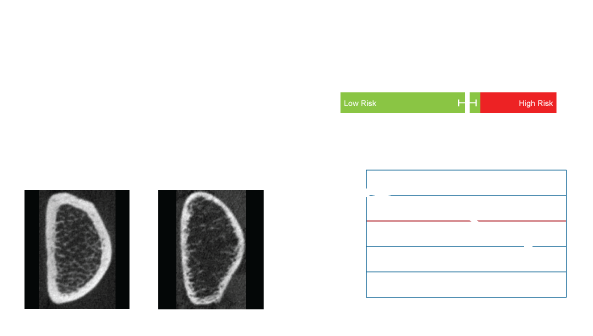
OssView® Bone Fragility software is under development to analyze InReach images at the distal radius to help evaluate the bone microstructure. OssView®is targeted to assess cortical and trabecular density, cortical thickness, and porosity to calculate a Structural Fragility Score (SFS). SFS, if cleared by FDA, could be used as complement to traditional bone mineral density (BMD) tests, allows clinicians an additional tool in assessment of non-osteoporotic women.
OssView® has received Breakthrough Device Designation from the US FDA Breakthrough Devices Program. Caution: OssView® is not cleared by the FDA for clinical use.
The OssView® Bone Fragility software is intended to be used as an aid in the clinical assessment of fracture risk in women 70 years and older. It is indicated to be used in women diagnosed as non-osteoporotic after a clinical exam using a bone densitometer. Ossview is CE Marking.
Structural Fragility Score - A New Measure for Quantifying Fracture Risk
The Structural Fragility Score (SFS), which we are developing to be delivered through OssView®, if authorized by FDA is for assessment of bone microstructure. SFS will be calculated from independent measurements of bone microstructure, specifically cortical porosity and trabecular density both extracted from a computed tomography (CT) image at the distal radius.
This technology is currently under development and is not yet cleared or approved by the U.S. FDA. Availability is subject to completion of development and applicable regulatory approvals. Regulatory approval and availability may vary by country.
OPERATIONAL BENEFITS
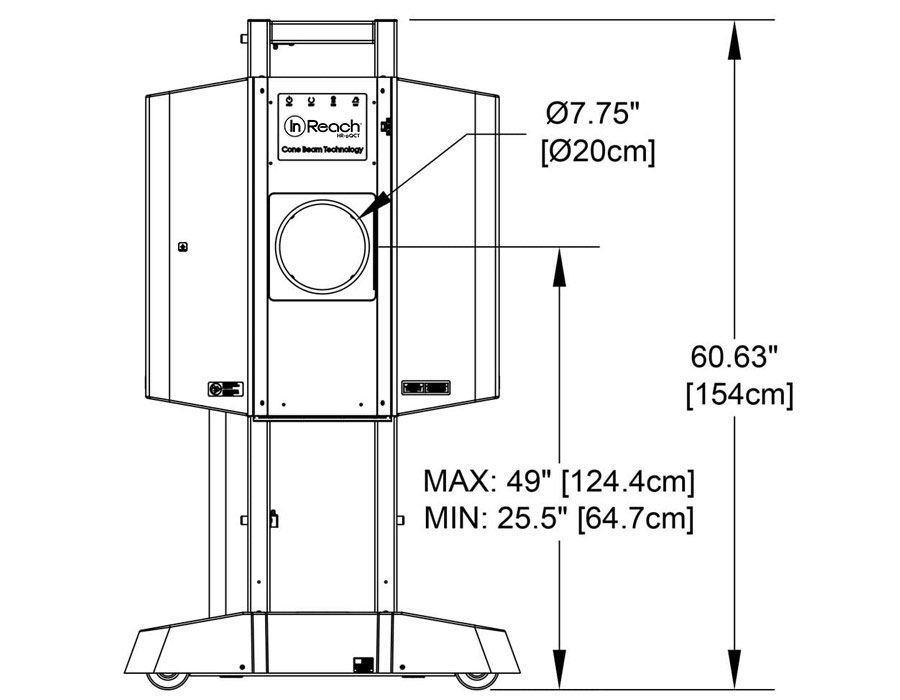
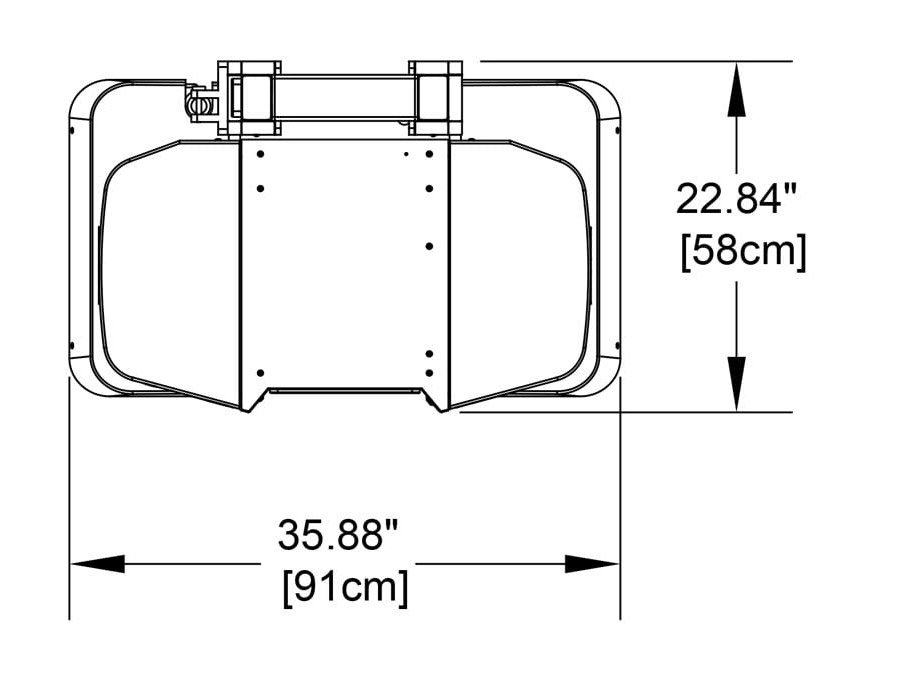
Compact
Systems can be sited in small spaces and next to existing imaging equipment. Cooling systems are not required. System weight is 300lbs/136kg.
Easy to Install
Systems fit through a standard doorway and plug into a standard wall outlet.
Self-Shielded
CurveBeam AI systems are self-shielded, and independent assessments have concluded that scatter radiation is close to zero once you reach a distance of six feet from the unit. Therefore, in most cases, minimal to no shielding modifications are required for CurveBeam AI system installations. However, only a qualified medical physicist may make this determination.